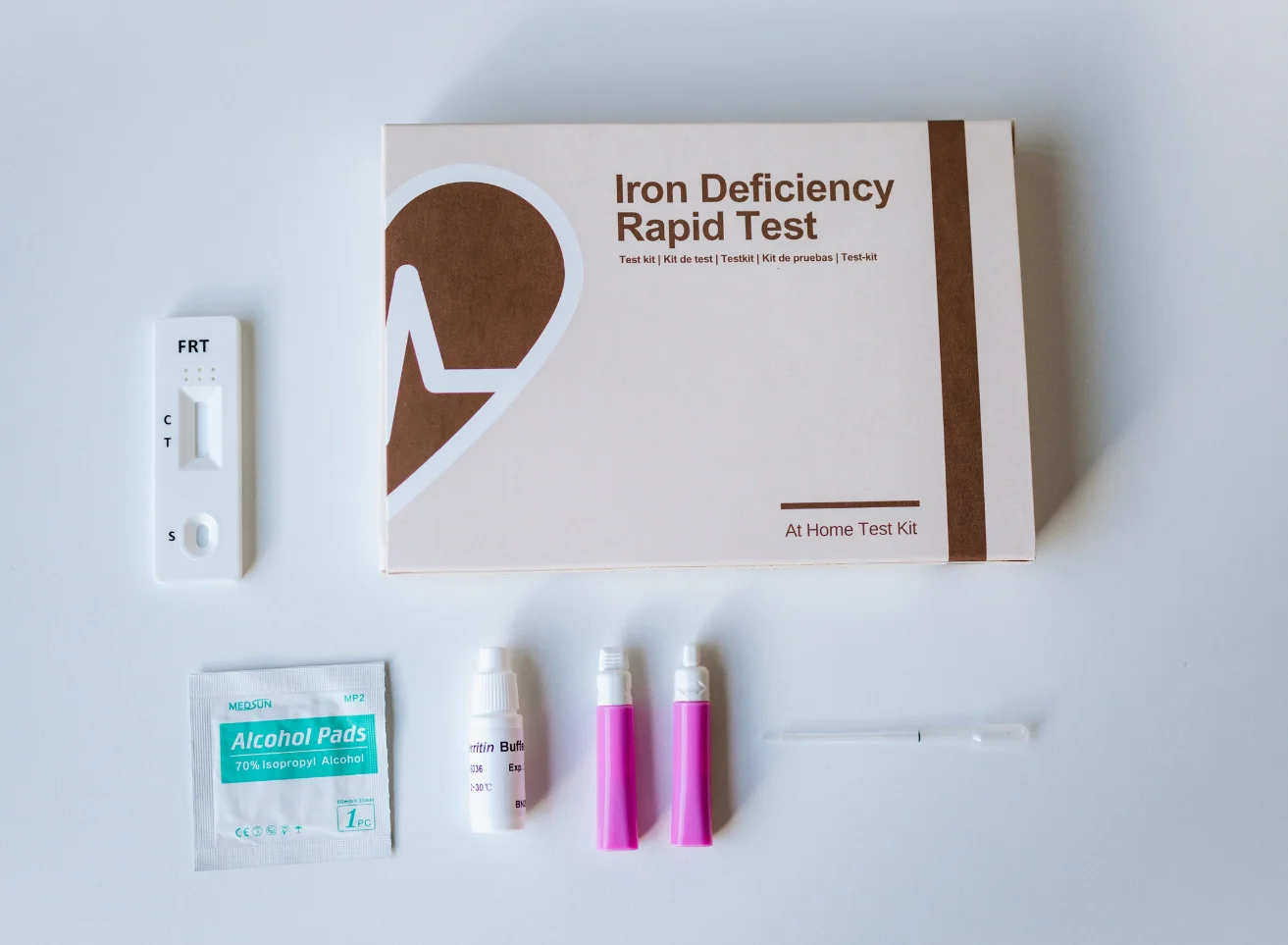
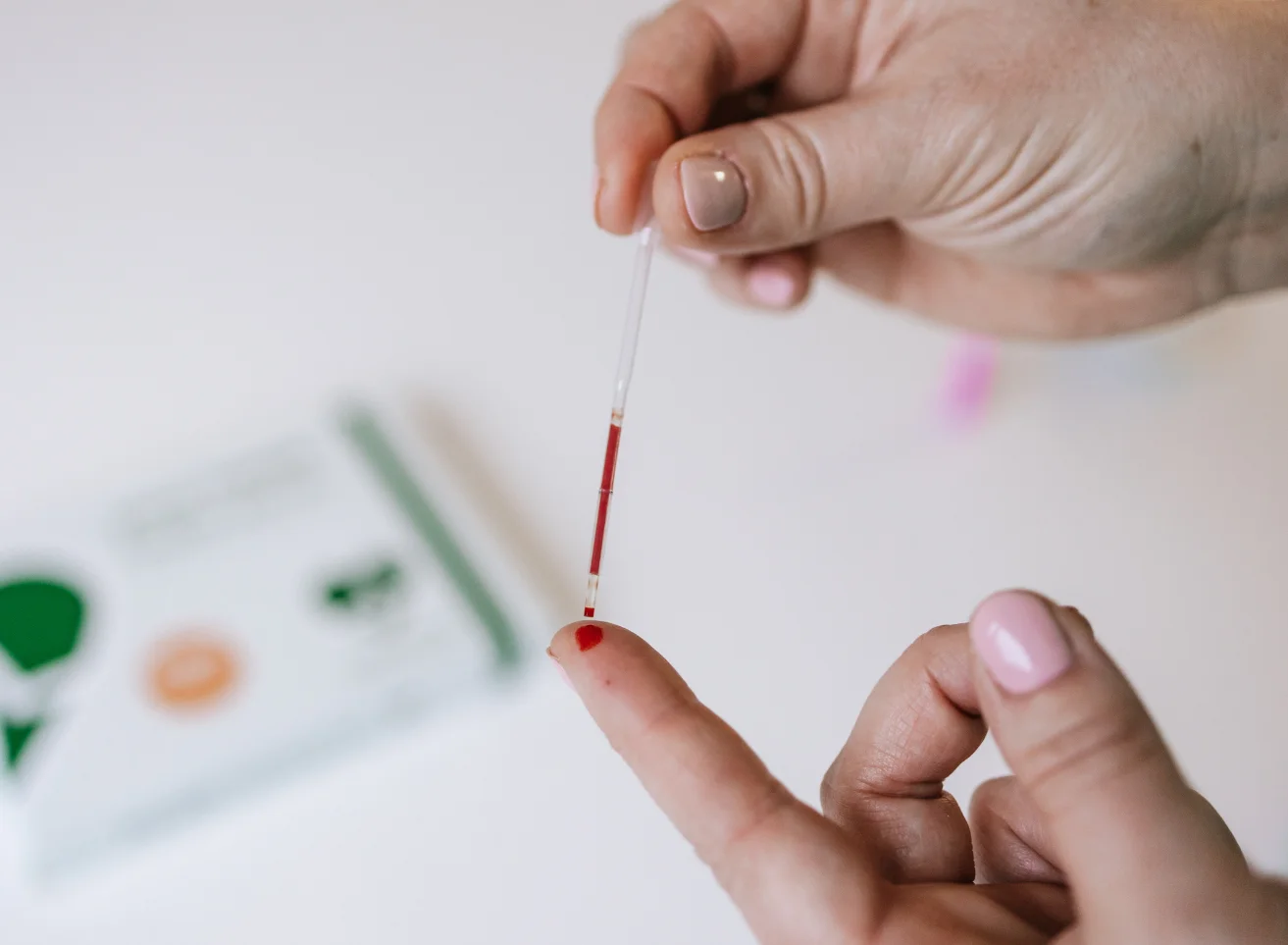
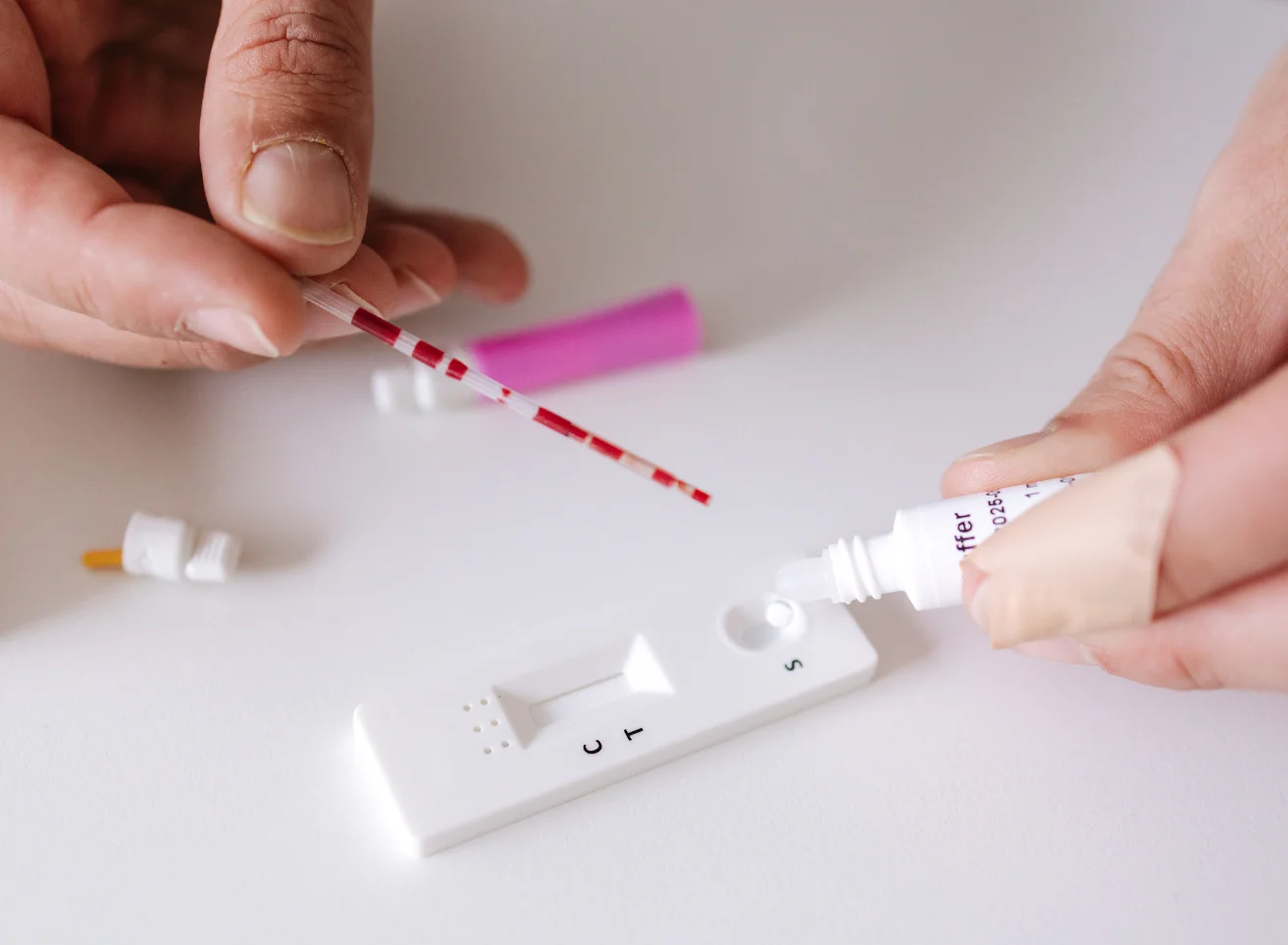
Ferritin is a large spherical protein consisting of 24 noncovalently linked subunits with a molecular weight of approximately 450 KD. One molecule of ferritin is capable of binding between 4000 and 5000 atoms of iron, making ferritin the major iron storage protein for the body (1). The concentration of ferritin is proportional to the total iron stores in the body, resulting in serum ferritin concentrations becoming a diagnostic tool in the evaluation of iron status.
In most normal adults, serum ferritin concentrations vary with age and sex. There is no sex difference until the onset of puberty, at which time ferritin concentrations rise, particularly in males. There is a significant positive correlation between age and serum ferritin concentrations in females but not in males (1-2).
Patients with iron deficiency anaemia have serum ferritin concentration of approximately one-tenth of normal subjects, while patients with iron overload (hemochromatosis, hemosiderosis) have serum ferritin concentrations much higher than normal. Studies also suggest that serum ferritin provides a sensitive means of detecting iron deficiency at an early stage (2).
References:
1) McPherson RA, Pincus MR eds: Henry’s Clinical Diagnosis and Management by Laboratory Methods. 21st ed. Elsevier Saunders; 2007:506
2) Cappellinin MD, Lo SF, Swickels DW: Hemoglobin, iron, bilirubin. In: Rafai N, Horvath AR, Wittwer CT, eds. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 6th ed. Elsevier Saunders; 2018:719-775